Abstract
Background: Intravenous (IV) iron is often used to treat iron deficiency anemia (IDA) in patients unresponsive to, or intolerant of, oral iron. IV iron has been associated with rare, but potentially serious acute hypersensitivity reactions (HSRs). The few randomized trials which assessed comparative risks of adverse events (AEs) with IV iron formulations are limited by small numbers and lack statistical power to identify differences in low frequency AEs such as HSRs. We have attempted to correct this deficit in the literature, by conducting a randomized double-blind clinical trial comparing two IV iron preparations.
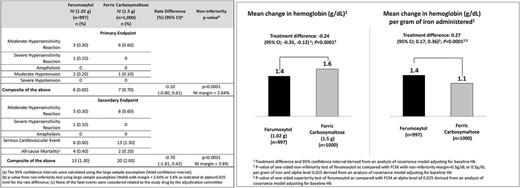
Aims: To evaluate the comparative safety and efficacy of standard courses of two IV iron preparations, ferumoxytol and ferric carboxymaltose (FCM), with the primary endpoint being the combined incidence of moderate to severe HSRs, including anaphylaxis, or moderate to severe hypotension. A secondary safety endpoint was the composite incidence of moderate to severe HSRs, including anaphylaxis, serious cardiovascular events, or death. Efficacy endpoints included change from baseline in hemoglobin (Hb) and change in Hb per gram of iron administered.
Methods: This randomized, double-blind, international, multicenter, trial (NCT02694978) included adults with IDA of any etiology excluding dialysis-dependent CKD (Hb <12.0 g/dL for females; <14.0 g/dL for males and transferrin saturation ≤20% or ferritin ≤100 ng/mL within 60 days of dosing) and a history of unsatisfactory response to or intolerance of oral iron. Randomization was 1:1 to ferumoxytol, administered as two 510 mg doses, or FCM, administered as two 750 mg doses both delivered as an IV infusion over at least 15 minutes on days 1 and 8. Patients were observed for at least 1 hour following the infusions. Follow-up visits were conducted at weeks 2 and 5. All events suggesting possible hypersensitivity or hypotension were reviewed and adjudicated by an expert, independent, blinded Clinical Events Committee (CEC) utilizing pre-specified criteria per protocol. All participants provided written informed consent before enrollment.
Results: 2014 patients were randomized at 129 sites in North America (US and Canada) and Eastern Europe (Latvia, Lithuania, Poland, and Hungary). The safety population included the 1997 randomized patients who received any amount of study drug (mean age 55.2 ±17.2 years, 76.1% female, mean baseline values: Hb 10.4 g/dL, transferrin saturation 13.9%, serum ferritin 57.4 ng/mL). The most common underlying causes of IDA included: chronic kidney disease (27%), abnormal uterine bleeding (25%) and gastrointestinal disorders (29%). Ferumoxytol demonstrated non-inferiority (NI) to FCM with respect to both the primary (ferumoxytol rate: 0.6%; FCM rate: 0.7%; treatment difference: -0.1% ; 95% confidence interval: -0.80% to +0.61%) and secondary safety endpoints (ferumoxytol rate: 1.3%; FCM rate: 2.0%; treatment difference: -0.7% ; 95% confidence interval: -1.81% to +0.42%) [TABLE]. Ferumoxytol demonstrated non-inferiority to FCM for both secondary efficacy endpoints (mean change in Hb from Baseline to Week 5, and mean change in Hb from Baseline to Week 5 per gram of iron administered), while additionally demonstrating a significantly greater increase in Hb per gram of iron administered [FIGURE]. Median increase from Baseline to Week 5 in serum ferritin was 182.2 ng/mL for ferumoxytol and 320.5 ng/mL for FCM.
Conclusion: While there is little debate as to the efficacy of IV irons on increasing hemoglobin concentrations in patients with IDA, the occurrence of rare but potentially serious acute HSRs continues to be a focus of concern. Very few well conducted trials have compared two or more IV iron formulations by evaluating HSR and hypotension as primary endpoints. In this study there was no significant difference between FCM and ferumoxytol in the composite endpoints of moderate to severe HSR, including anaphylaxis, or moderate to severe hypotension, serious cardiovascular events or all-cause mortality.
Auerbach: AMAG Pharma, Pharmacosmos, Luitpold: Consultancy; AMAG Pharma, Pharmacosmos: Research Funding. Strauss: AMAG pharmaceuticals: Employment, Equity Ownership. Macdougall: AMAG: Consultancy, Honoraria, Research Funding; Pharmacosmos: Honoraria; Vifor Pharma: Consultancy, Honoraria, Research Funding, Speakers Bureau; Akebia: Consultancy; GlaxoSmithKline: Consultancy; Astellas: Honoraria, Research Funding; FibroGen: Consultancy, Speakers Bureau. Bernard: AMAG: Employment. Kaper: AMAG Pharmaceuticals, Inc.: Employment. Chertow: AMAG: Consultancy; Akebia: Consultancy; Amgen: Consultancy, Research Funding; Keryx: Consultancy. Li: AMAG Pharmaceuticals: Employment, Equity Ownership. Trochanov: AMAG Pharmaceutical,Inc: Employment, Other: Company stock. Dahl: AMAG Pharmaceuticals. Inc.: Employment, Equity Ownership. Krop: AMAG Pharmaceuticals: Employment, Other: Stock in company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal